
MeKo Presents PolyMediX® Polymer Tubes
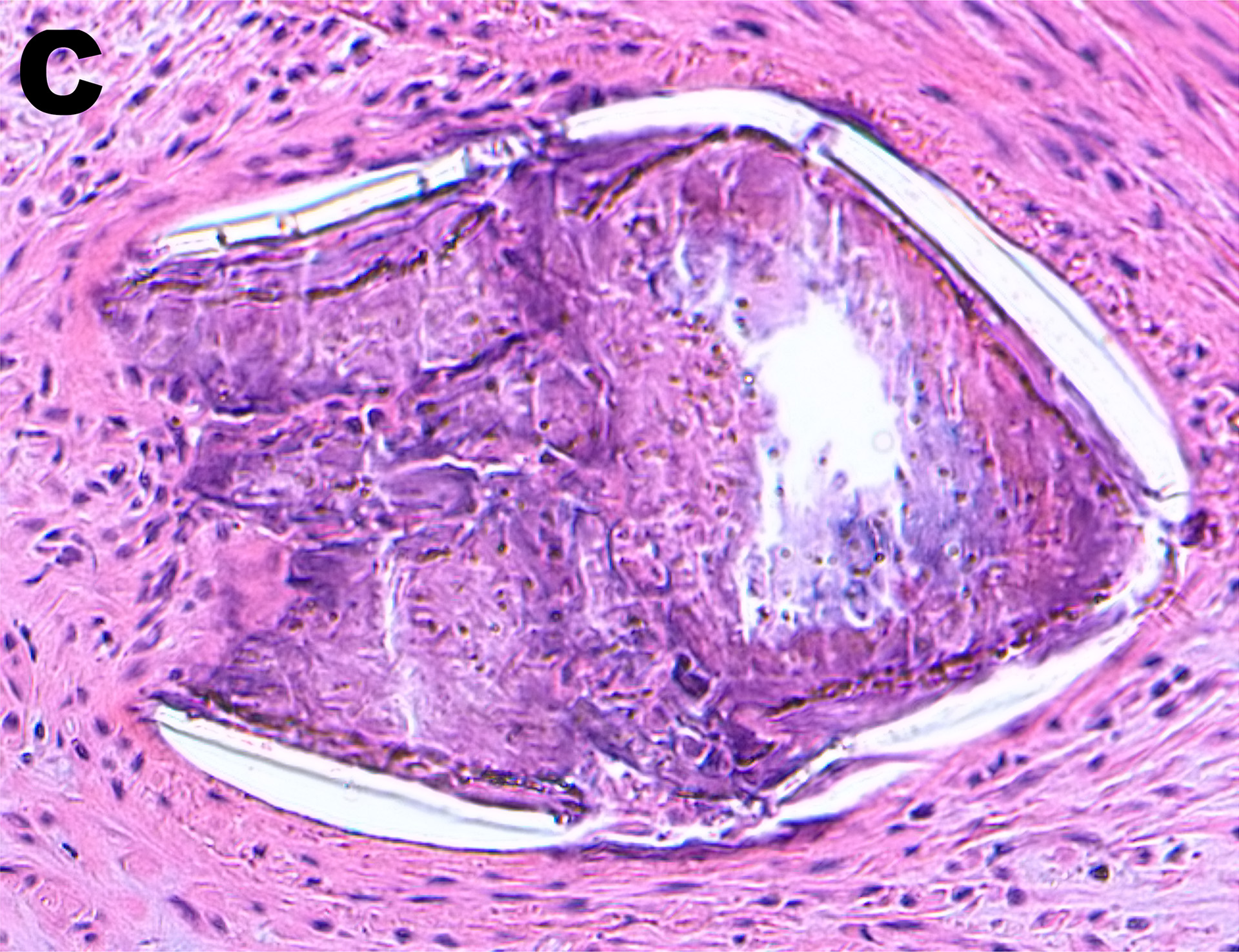
MeKo is known as a contract manufacturer of medical components with particularly tight tolerances, such as stents or heart valve frames. At this year’s COMPAMED / MEDICA trade fair, MeKo will present PolyMediX®, its innovative polymer tubing used for the manufacturing of high-precision bioresorbable implants.
The high-precision PolyMediX® tubes are made using an innovative manufacturing process developed by MeKo. Thanks to the particularly gentle manufacturing process, PolyMediX® enables direct and non-destructive integration of drugs into the tube walls.
In this case, “poly” has a double meaning. It stands for “polymer”, but it also refers to the multiple possibilities offered by this manufacturing process. In addition to the ability to use a wide range of different polymers or different combinations of polymers and active agents for “medi”cal implants, this manufacturing process also makes it possible to integrate multiple active agents “X” or set an active agent gradient between the outside and the inside of the tube wall. With PolyMediX®, MeKo makes it possible to manufacture implants from polymer materials that are perfectly adapted to individual customer requirements.
In 2011, MeKo presented RESOLOY®, the resorbable magnesium alloy for scaffolds, which is already being used for resorbable implants today. With the PolyMediX® platform, MeKo is expanding its repertoire in the field of bioresorbable materials and utilizing its unique expertise in the production of bioresorbable implants.










 Read more about our certifications
Read more about our certifications
